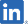| PIPELINES | TARGET INDICATION |
AREA | PRE- CLINICAL |
CLINIAL STUDY | NDA | APPROVAL /LAUNCH |
PROGRESS | PARTNER | ||
|---|---|---|---|---|---|---|---|---|---|---|
| PHASE 1 | PHASE 2 | PHASE 3 | ||||||||
|
SP-05 MORE INFO |
Colorectal Cancer (CRC) | Japan |
|
|
|
|
|
|
Decision to resume development. |
ー |
SP-05
In August 2020, Solasia acquired the rights to SP-05 (arfolitixorin) from Isofol Medical AB for
Japan.
SP-05 is a product pipeline for an increase in the antitumor efficacy of
fluorouracil.
Isofol executed several clinical studies in Europe, and the results from a phase
II study indicate that SP-05 has a potential to increases the antitumor effect of fluorouracil in
patients with advanced colorectal cancer.
Development Status: Clinical study planning
In December 2018, a global phase III study has been initiated by Isofol in the U.S., Canada, Europe,
Australia and Japan.
Nov. 2022 Phase III clinical trials are completed; primary endpoints not
met; development suspended.
March 2024: Decision to resume development.
Colombia and Peru
have already been submitted for application.
Expected Target Indications
Increase in the antitumor efficacy of fluorouracil
Arfolitixorin is a new drug candidate being developed to treat colorectal cancer (CRC). Other
potential indication of arfolitixorin in combination with fluorouracil is including the treatment
for pancreatic cancer, breast cancer, stomach cancer, and head and neck cancers.
The
combination use of DNA synthesis inhibitor, fluorouracil (5-FU), and folate-based prodrugs,
leucovorin (LV) and levoleucovorin (l-LV), is standard of care chemotherapy for advanced colorectal
cancer, and folate analogs are used to increase the antitumor effect of fluorouracil
(5-FU).
Unlike leucovorin (LV) and levoleucovorin (l-LV); which must be metabolized into MTHF
([6R]-5, 10-methylenetetrahydrofolate) in the body in order to be effective in the treatment of
cancer, the active substance in arfolitixorin is MTHF. This means that no metabolic activation is
required and arfolitixorin consequently has the potential to achieve a more powerful antitumor
effect for all patients in combination with fluorouracil (5-FU) treatment.