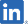| PIPELINES | INDICATION | AREA | PRE- CLINICAL |
CLINIAL STUDY | NDA | APPROVAL /LAUNCH |
PROGRESS | PARTNER | ||
|---|---|---|---|---|---|---|---|---|---|---|
| PHASE 1 | PHASE 2 | PHASE 3 | ||||||||
|
DARVIAS® (SP-02) MORE INFO |
Peripheral T-Cell Lymphoma (PTCL) | Japan |
|
|
|
|
|
|
Launched in August 2022 Began searching for additional indications |
Nippon Kayaku:
|
| South Korea, Taiwan, Hong Kong |
|
|
|
|
|
|
Phase II (pivotal) study completed Out-licensing activities ongoing | |||
| South America |
|
|
|
|
|
|
Preparations to file for approval underway in each country based on approval granted in Japan(Colombia and Peru have already filed.) | |||
| China |
|
|
|
|
|
|
Development strategy being drafted based on US study data and approval in Japan; out-licensing activity ongoing | |||
| US |
|
|
|
|
|
|
Early Phase II study completed; out-licensing activity ongoing | |||
| EU |
|
|
|
|
|
|
NPP strategy being launched | |||
SP-02
Solasia acquired the rights to darinaparsin (SP-02) from Alaunos Therapeutics (Nasdaq: TCRT) for
worldwide rights.
Darinaparsin, an organoarsenic compound with anticancer activity, is a
novel mitochondrial-targeted agent being developed for the treatment of various hematologic and
solid tumors. The proposed mechanism of action of the drug involves the disruption of mitochondrial
function, increased production of reactive oxygen species, and modulation of intracellular signal
transduction pathways. Darinaparsin is believed to exert anticancer effect by inducing cell cycle
arrest and apoptosis. Darinaparsin has been granted orphan drug designation in the U.S. and Europe
as a treatment of peripheral T-cell lymphoma (PTCL).
In June 2020, darinaparsin achieved
the primary endpoint (antitumor effect) in the Asian multinational phase 2 clinical study in
patients with relapsed or refractory PTCL in Japan, South Korea, Taiwan, and Hong
Kong.
In June 2022, Solasia has been approved a New Drug Application (NDA) for
darinaparsin as a treatment for relapsed or refractory peripheral T-cell lymphoma to the Ministry of
Health, Labour and Welfare (MHLW) in Japan.
Development Strategy: Approval (Japan), Preparation for NDA filing (South Korea, Taiwan, Hong Kong)
Expected Target Indications
Peripheral T-Cell Lymphoma (PTCL)
PTCL is a type of malignant lymphoma. Malignant lymphoma is largely classified into Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL), and non-Hodgkin lymphoma is further divided into two main categories of B-cell lymphoma and T-cell lymphoma. PTCL is a subtype of T-cell lymphoma with a relatively high incidence rate among other subtypes of T-cell lymphoma. No standard treatment has been established for PTCL, and the National Comprehensive Cancer Network guidelines recommend as front-line treatment participation in clinical trials or multidrug chemotherapy such as CHOP therapy. For relapsed or refractory PTCL patients, in addition to participation in clinical trials, multidrug chemotherapy or recently approved drugs are recommended. However, as with the newly diagnosed cases, no standard therapy has been established. As prognosis for PTCL may be poor and its treatment difficult, it is one of the diseases with high unmet medical needs for which the development and introduction of new therapies and drugs are much anticipated.