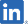| PIPELINES | INDICATION | AREA | PRE- CLINICAL |
CLINIAL STUDY | NDA | APPROVAL /LAUNCH |
PROGRESS | PARTNER | ||
|---|---|---|---|---|---|---|---|---|---|---|
| PHASE 1 | PHASE 2 | PHASE 3 | ||||||||
|
Sancuso®(SP-01) MORE INFO |
Chemotherapy Induced Nausea and Vomiting (CINV) | China |
|
|
|
|
|
|
Launched in 2019 Preparing to apply for change of manufacturing facility |
Lee’s Pharm: |
SP-01
In May 2008, Solasia acquired rights to SP-01 (Sancuso®) from Strakan International Ltd. (currently, Strakan International S.à.r.l.) for China and other countries in Asia. Sancuso® is an extended release transdermal system, delivering the anti-emetic, granisetron, steadily into the patient’s bloodstream over several days. Transdermal delivery is especially beneficial to patients receiving chemotherapy who cannot swallow medicines due to nausea or mucositis. Clinical guidelines recommend the use of the 5-hydroxytryptamine sub-type 3 (5-HT3) receptor antagonists in the prevention of chemotherapy-induced nausea and vomiting (CINV). Granisetron is a 5-HT3 receptor antagonist with well-established efficacy against CINV, and Sancuso® is the world’s first and only transdermal patch of the 5-HT3 receptor antagonist. Sancuso® was approved by the U.S. Food & Drug Administration (FDA) in September 2008 for the prevention of CINV in patients receiving moderately and/or highly emetogenic chemotherapy for up to 5 consecutive days. Besides the U.S., Sancuso®was approved many countries and areas including U.S., England, Germany, Netherlands, Denmark, South Korea, Taiwan.
Development Status (China) : Launched in China
A clinical pharmacology study and a randomized, double blind, oral granisetron controlled clinical study in
China completed in 2014. New Drug Application (NDA) was submitted in June 2014, and SP-01
(Sancuso®) was approved by the China National Drug Administration (CNDA) in July 2018.
In
March 2019, Sancuso® has been launched.
Target Indications
Chemotherapy-Induced Nausea and Vomiting (CINV)
Nausea and vomiting are problematic adverse events for patients receiving chemotherapy. Inadequately controlled CINV can precipitate a number of medical complications that may prove life-threatening, including dehydration and electrolyte imbalance, malnutrition or aspiration pneumonia. These complications may lead to extended hospitalization, with the associated burden on nursing time and pharmacy resources and overall cost implications. CINV has a considerable impact on aspects of the patient’s quality of life, as well as those of the family and caregivers. For some regimens such as high-dose cisplatin, which has a strong emetic effect, CINV can affect as many as 90% of patients making patient compliance a potential problem. The distress resulting from these symptoms can escalate over time and interfere even with the most effective antitumor therapy: as a consequence of failing to control CINV, patients may be less compliant with their chemotherapy. CINV is usually classified as either acute (up to 24 h) or delayed (post 24 h), although it is recognized that this classification is partly arbitrary. The incidence of nausea and vomiting during the acute phase is probably a predictor of the incidence of delayed CINV. Poorly controlled nausea and vomiting in previous chemotherapy increases the likelihood of CINV. Control of nausea and vomiting in patients receiving chemotherapy is, therefore, an important objective of supportive care in cancer chemotherapy.