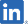| PIPELINES | INDICATION | AREA | PRE- CLINICAL |
CLINIAL STUDY | NDA | APPROVAL /LAUNCH |
PROGRESS | PARTNER | ||
|---|---|---|---|---|---|---|---|---|---|---|
| PHASE 1 | PHASE 2 | PHASE 3 | ||||||||
|
episil® oral liquid(SP-03) MORE INFO |
Pain associated oral mucositis [Medical Device] | Japan |
|
|
|
|
Launched in 2018 |
Meiji Seika Pharma: Japan
Changchun GeneScience Pharmaceutical:
Synex: |
||
| China |
|
|
|
|
Launched in 2019 | |||||
| South Korea |
|
|
|
|
Launched in 2020 | |||||
SP-03
In March 2015, Solasia acquired exclusive commercialization rights in Japan and China to SP-03 from Camurus, also acquired South Korea right in August 2018. SP-03(episil®) is a liquid for protecting and dressing of oral lesions and categorized in non-absorptive liquid medical device. Upon contact with the aqueous fluid in the oral mucosa, episil® forms a protective film within a few minutes, which acts as a mechanical barrier and provides relief of pain suffered from oral mucositis. episil® was developed using the award-winning Camurus proprietary technology FluidCrystal®. Clinically demonstrated, episil® has been shown immediate (within minutes) and effective pain relief which lasted 8 hours*. episil® is supplied as a ready-to-use, pocket-sized device helping patients improve their quality of life while undergoing chemotherapy and/or radiotherapy. FluidCrystal® was awarded the “Best innovation in formulation” prize at the CPhI Worldwide in 2013.
* Hadjieva, T et al. Treatment of oral mucositis pain following radiation therapy for head-and-neck cancer using a bioadhesive barrier-forming lipid solution. Support Care Cancer 2014, 22:1557–1562
Development Status: Launched in Japan, China, and South Korea
In Japan, episil® was approved in July 2017, launched by Solasia’s commercialization
and promotion partner, Meiji Seika Pharma Co., Ltd. in May 2018.
In China,
episil® was approved in February 2019, and launched in July 2019.In the Chinese market,
Changchun GeneScience Pharmaceutical Co.,Ltd. has been conducting sales activities since March 2025.
In South
Korea, episil® was approved in October 2019, and has been launched in September 2020.