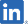| PIPELINES | TARGET INDICATION |
AREA | PRE- CLINICAL |
CLINIAL STUDY | NDA | APPROVAL /LAUNCH |
PROGRESS | PARTNER | ||
|---|---|---|---|---|---|---|---|---|---|---|
| PHASE 1 | PHASE 2 | PHASE 3 | ||||||||
|
SP-04 MORE INFO |
Chemotherapy Induced Peripheral Neuropathy (CIPN) | Japan etc. |
|
|
|
|
|
|
Pre-clinical study in taxane-induced peripheral neuropathy |
Maruho: |
SP-04
In 2017, Solasia acquired the rights to SP-04 (PledOx) from PledPharma AB (currently Egetis Therapeutics AB) for Japan, China, Hong Kong, Macau, South Korea and Taiwan. SP-04 is a product pipeline for Chemotherapy Induced Peripheral Neuropathy. The side-effects of chemotherapy can lead to a reduction of the planned dose or, in worst case, treatment discontinuation. Unfortunately, it appears that the chemotherapy can induce permanent nerve damage. Patients may, for example, experience discomfort and numbness in the hands and feet, difficulty with balance with risk of falling and problems with sensation that can last for the rest of their lives.
Development Status: Pre-Clinical development
Under Pre-clinical study in taxane-induced peripheral neuropathy
Expected Target Indications
Chemotherapy Induced Peripheral Neuropathy (CIPN)
Cancer chemotherapy has side effects such as nausea, vomiting, and stomatitis. Chemotherapy-induced peripheral neuropathy (CIPN) is another major side effect caused by Taxanes (paclitaxel, etc.), platinum-based agents (oxaliplatin, cisplatin, etc.), Vinca alkaloids*. However, there are currently no drugs approved for the indication of CIPN (based on Solasia survey).
* Reference: Ministry of Health, Labor and Welfare “Corresponding manual for severe side effects disease Peripheral neuropathy